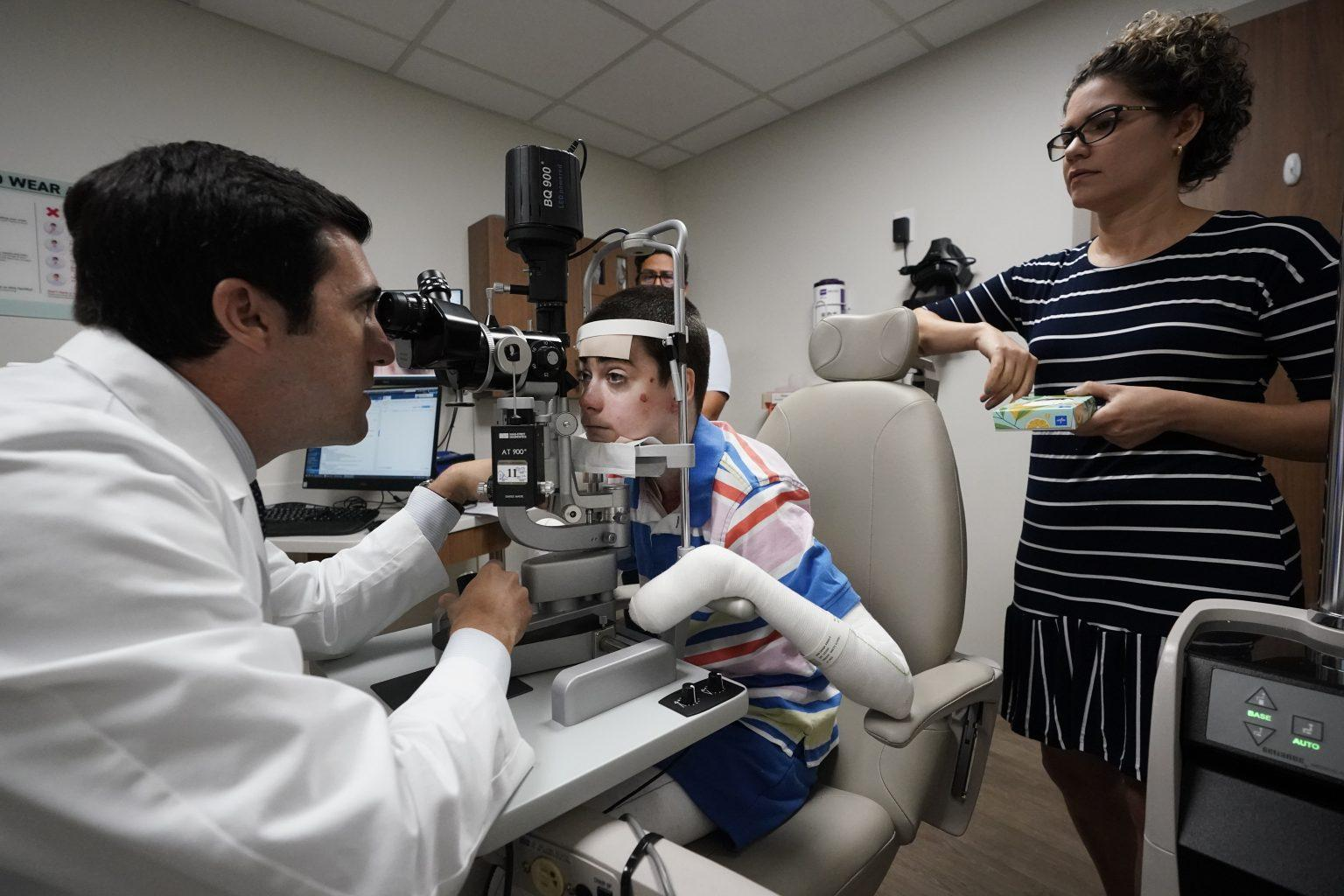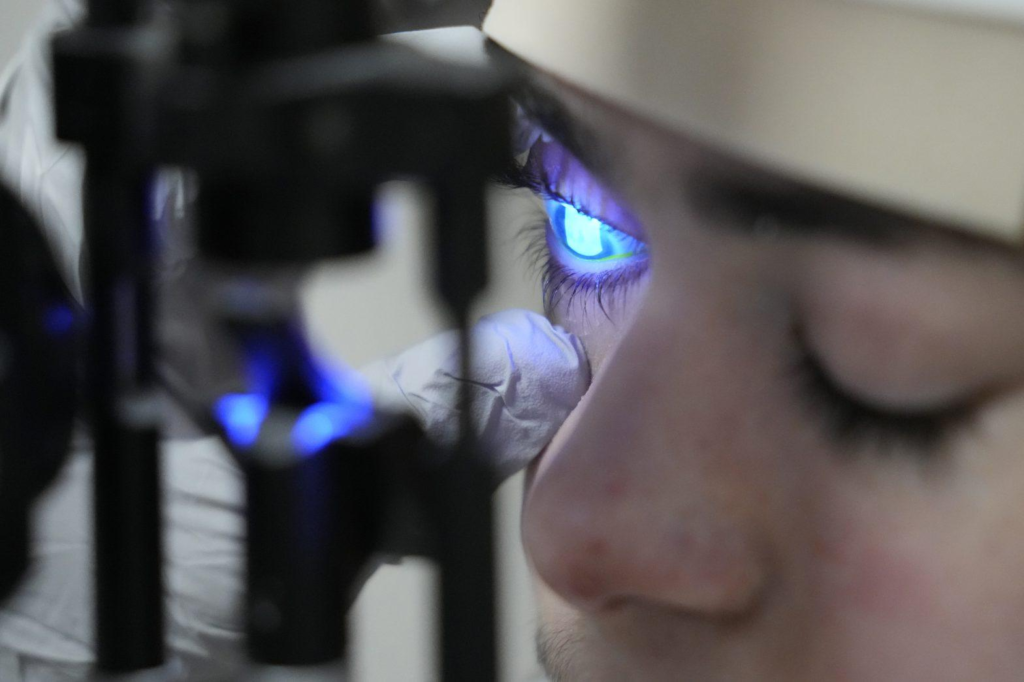
Dr. Alfonso Sabater retrieved two photographs of Antonio Vento Carvajal’s eyes. One showed cloudy scars covering both eye bulbs. The other, taken after months of gene therapy administered through eye drops, revealed no scars in either of the two eyes.
Antonio, who was legally blind for a significant part of his 14 years, can see again.
The teenager was born with dystrophic epidermolysis bullosa, a rare genetic condition that causes blisters all over the body, including the eyes. However, his skin improved when he enrolled in a clinical trial for the world’s first localized gene therapy. This gave Dr. Sabater an idea: What if it could be adapted for Antonio’s eyes?
This realization not only helped Antonio but also opened the door to similar therapies that could potentially treat millions of people with other ocular conditions, including common disorders.
Antonio’s mother, Yuni Carvajal, cried thinking about what Dr. Sabater did for her son.
“He was there for everything,” she said in Spanish to The Associated Press during a visit to the Bascom Palmer Eye Institute at the University of Miami Health System. “He’s not just a good doctor but also such a good person, and he gave us hope. He never gave up.”
The family came to the U.S. from Cuba in 2012 on a special visa that allowed Antonio to receive treatment for his condition, which affects around 3,000 people worldwide. He underwent surgeries to remove scar tissue from his eyes, but it kept growing back. Antonio’s vision was constantly deteriorating, and it eventually worsened to the point where he didn’t feel safe walking.
Sabater didn’t have answers and tried to reassure the boy, “I will find a solution. I just need a little time. I’m working on it.”
“Yes, I know you’ll make it,” Sabater recalls Antonio saying. “That gave me the energy to keep going.”
At some point, Carvajal told Sabater about the experimental gene therapy gel for Antonio’s skin condition. She reached out to the pharmaceutical company Krystal Biotech to see if it could be adapted for the boy’s eyes.
Suma Krishnan, co-founder and president of research and development at the Pittsburgh-based company, said the idea made sense and “it doesn’t hurt to try.”
Antonio’s condition is caused by mutations in a gene that contributes to the production of a protein called collagen 7, which anchors both the skin and the cornea. The therapy, called Vyjuvek, uses a disabled herpes simplex virus to deliver functional copies of this gene. The eye drops use the same fluid as the skin version, just without the added gel.
After two years, including trials of the drug in mice, the team received “compassionate use” approval from the U.S. Food and Drug Administration and clearance from the University and Hospital review boards. Last August, Antonio underwent eye surgery on his right eye, after which Sabater started giving him the eye drops.
Krishnan said they were cautious, closely monitoring to ensure safety.
Antonio’s eye recovered from the surgery, the scars did not return, and there was significant improvement each month, according to Sabater. Recently, doctors measured Antonio’s vision in his right eye at nearly perfect 20/25.
Source: Ethnikos Kyrix